| Please note, head-to-head prospective clinical trials are needed to further understand the clinical benefits that inhaled extrafine formulation therapies may provide in comparison to non-extrafine formulations.1-2 |
In this article, we’ll explore in detail the small airways, their contribution to the lungs, small airways disease (SAD) and crucially, their involvement in the aetiology and treatment of asthma and COPD.
Small airways are a key player in the anatomy of healthy lungs
The structure of our respiratory system is critical to how it works. . The lungs are arranged in a branching system, segmentally dividing from the trachea (generation 1) down to the alveoli (generation 23) (Figure 1). As the trachea repeatedly branches into smaller airways, the speed of airflow decreases, and the total cross-sectional area for capturing oxygen increases. The small airways account for 98.8% of the total lung volume.3 This helps ensure there is time for optimal gas exchange with every breath.4-5
The different airways within the respiratory system
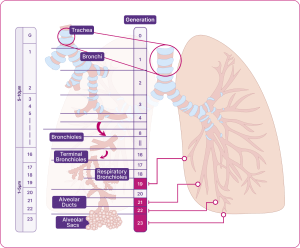
Adapted from Usmani et al. 2021.5
All airways can be considered as either large or small, based on size – both are equally important for healthy lung function.5-6
- Large airways are greater than 2mm in diameter (e.g. the trachea, the bronchi).
- Small airways, beginning at Generation 8, lead to the alveoli and have an internal diameter of less than 2mm. (e.g. the bronchioles, the terminal bronchioles, the respiratory bronchioles, and the alveolar ducts and sacs).6
In both asthma and COPD, the small airways are major sites of inflammation and airflow resistance, affecting normal lung function4,6
Disease in the small airways can often progress without obvious symptoms, leading to many referring to this region as the lung’s ‘silent zone’.5,7-8 Yet, the silence is misleading; the small airways have a significant impact on airflow resistance and disease control.6
Small airway disease (SAD): a common feature of asthma and COPD4,8
Small airways disease is gaining recognition as an important feature in asthma and COPD and is defined as any physiological abnormality seen in the small airways.4,8 In-fact ~90% of patients with asthma and symptomatic COPD have small airways disease:5,8,9
- Adult asthma: in a prospective cohort study (N=773), 91% of patients with asthma had small airways disease.
- COPD: in an observational study (N=202), 93% of patients with symptomatic COPD (CAT score ≥10) had small airways disease.
Detecting and quantifying small airways disease remains a major clinical challenge.5 Small airways disease can be present even in patients who appear clinically stable, or who appear to have normal lung function.10-11 Traditional imaging techniques lack the sensitivity to capture early-stage disease, and spirometry, the gold standard for respiratory disease diagnoses also has its limitations.5
The disease may be evident long before spirometry or imaging reveals any abnormalities, with studies showing that up to 75% of the small airway cross-sectional area must be destroyed before airflow limitation is detectable by spirometry. By this stage, the disease is already well established.3
Small airway disease in asthma
Asthma affects the whole bronchial tree, including the small airways. It is characterised by hypersensitivity, obstruction and inflammation. Airway obstruction is characterised by the tightening of the airway muscles (bronchoconstriction) and mucus production.1,8,12 Obstruction and inflammation may also trigger myofibroblasts to increase the thickness of the inner walls of the airways, narrowing them further and reducing the area for gas exchange.12
SAD in asthma is associated with:3,6,8,10,13-15
- chronic airway inflammation
- airway hyperresponsiveness
- airway remodelling (i.e. changes to the shape/structure of the airways)
- total airflow resistance
- increased risk of symptoms and exacerbations (high vs low SAD scoring group)8
- breathlessness
- lower quality of life (QoL) and more frequent healthcare use (high vs low SAD score)
- suboptimal asthma control
The presence of SAD has been shown to be consistently highest in those with the highest severity of asthma.8
Airway hyperresponsiveness (AHR), the exaggerated tendency of the airways to constrict in response to stimuli that have little to no effect in healthy individuals, is also a defining feature in asthma.16 SAD is strongly associated with AHR in patient with mild-moderate asthma.15-17 This heightened responsiveness translates into recurrent symptoms such as wheezing, breathlessness, and cough, while also driving exacerbation risk and disease progression.
Small airway disease in COPD
COPD, or chronic obstructive pulmonary disease, is a collective term used for several respiratory conditions affecting the lungs – including emphysema and bronchitis.18
Damage to the cilia in the bronchus is a feature of COPD – this affects the normal clearing of mucus and external substances from the airways; meaning more gets through to lower parts of the lungs.5,19 Additionally, the anatomy and function of the small airways in slowing the airflow may mean they are exposed to any harmful particles for longer periods than larger airways.4
Small airways disease has been an established as a key contributor to airway resistance in COPD for over 50 years.4-5 Evidence for SAD in COPD has been validated in physiological, imaging and histopathological studies.4
SAD is often evident before the onset of symptoms or changes in spirometry or imaging findings in COPD.5
It is suggested that SAD occurs early in the aetiology of COPD and may happen before damage to the alveoli, known as emphysema. It is thought that emphysema may also worsen SAD in return, in a damaging feedback cycle.4-5
SAD in COPD is associated with abnormalities such as airway remodelling and wall thickening, increased small airway resistance, mucus plugging and immune infiltration in submucosa of small airways (i.e. inflammatory response).4-5
There are a number of clinical consequences which result from SAD and these can include:5,20
- Increased risk of symptoms and exacerbations
- Disease progression
- Poor lung function
- Impaired quality of life
- Lung hyperinflation, at rest and during exercise (i.e. larger-than-normal lungs, as a result of trapped air)
The cycle of the pathophysiological changes in SAD
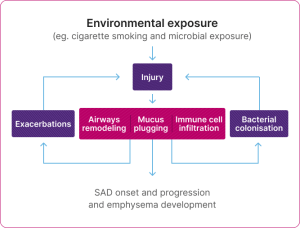
Adapted from Usmani et al. 2021.5
A key consequence of small airways disease in COPD is hyperinflation, which is the abnormal increase in lung volume caused by trapped air.21-23 Hyperinflation is defined as an increase in residual volume (RV) or increase in the ratio of RV compared to total lung capacity (TLC) beyond the 95th centile.24 As damage to the alveoli leads to loss of elasticity, patients are unable to fully empty their lungs during exhalation. 22-23
Hyperinflation can also have adverse effects on the cardiovascular system and can increase the risk of cardiovascular events, including increased pulmonary hypertension and congestive heart failure. 24-29 In addition to causing hyperinflation, obstruction of the small airways has also shown to increase risk of early death in patients with COPD.30-31
Cardiovascular disease (CVD) affects half of patients with COPD, and the diagnosis of COPD nearly doubles the risk of a heart attack.32-33
Timely optimisation of treatment
Every exacerbation can lead to a poorer prognosis, highlighting the need for early optimisation in patients with asthma and COPD.34-35
- For COPD:
- There is a 50% mortality rate within 3.6 years of a patient experiencing their first severe exacerbation, increasing to 75% after 7.7 years.36
- Timely optimisation of symptomatic patients with medications that target the small airways may slow disease progression, improve symptoms, and help patients maintain their daily activities.5
- For asthma:
- Inflammation driving asthma exacerbations leads to permanent structural damage in the lungs, known as airway remodelling, with every exacerbation leading to lung function decline, highlighting the need for timely optimisation.34
Treatments targeting the small airways may be particularly beneficial in the management of both adult asthma and COPD.5,10
Extrafine formulations can reach both large and small airways1,4,37
Both the large and small airways are important targets for the treatment of asthma and COPD. 4,6, 8,37
Treatments for these conditions can differ in both treatment class and in molecule size.38-40 The size of the particles, measured by mass median aerodynamic diameter (MMAD), that deliver medication into the lungs can impact how far they travel and how widely they are dispersed.1,4,37 In general, clinical studies suggest the following distinction:1,4,37
- large particles: can reach oropharynx, trachea, and the upper bronchial branches
- small particles: can also reach the distal airways
Extrafine particles, defined as having an MMAD of <2 µm, can distribute uniformly across the entire bronchial tree, including the peripheral airways.1,38
Airways of the respiratory system
Diagram indicating the MMAD of Fostair® (beclometasone/formoterol) and Trimbow®(beclometasone/formoterol/glycopyrronium)
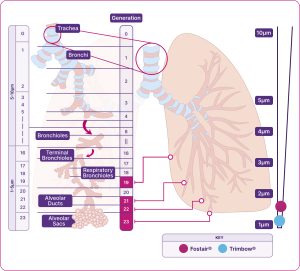
Adapted from Usmani et al. 2021.1,5
Trimbow and Fostair have on average much smaller aerosol particles than non-extrafine formulations. For the beclometasone component, this results in a more potent effect and a 2.5x lower nominal steroid dose compared to non-extrafine formulations42,46,48
Relative scale (not actual size).
*Average particle size of all components.
The only portfolio of products with an extrafine formulation, available in a range of devices and strengths42-48,61
Trimbow and Fostair are designed to reach and treat the large and small airways1,42-55

The importance of finding the right inhaler for each patient56
Many other factors, beyond formulation, can affect how well a treatment is delivered throughout the large and small airways in the lungs.56 Other factors that could have an impact on the success of a patient’s treatment include:1,6,56-58
- device type, including the use of multiple vs single inhaler devices
- patient age (also impaired cognition, coordination, and morbidity)
- improper inhaler technique (related to a 50% increased risk of hospitalisation in COPD)
- appropriate training in the use of inhaler device.
When choosing an inhaler with a patient, it is important to consider their preferences, lung function and their inhaler technique.56 A suitable device can improve adherence, health outcomes and QoL.39 An inappropriate one may lead to poor health outcomes and increased risk of exacerbations.56,58-60
Our Patient Support and Education and Events pages include expert videos and action plans to help you support patients with their technique and adherence.









