Uncontrolled asthma persists as a major burden in the UK.1-3 Findings from a newly published real-world study of Fostair NEXThaler 100/6 highlight its role in improving asthma management in everyday practice.4
The pervasive burden of uncontrolled asthma in the UK1–3
Uncontrolled asthma is not only a clinical challenge; it is a significant burden impacting the UK’s healthcare system and, more importantly, patients’ lives.1–3
Nearly half (48%) of patients with asthma struggle with uncontrolled symptoms annually, requiring oral steroids or use of multiple reliever inhalers – indicating suboptimal disease management.3
The consequences extend beyond the struggle with symptoms to life-threatening events.1,2 Asthma exacerbations account for over 65,000 hospital admissions each year, placing substantial strain on the healthcare system.2 And sadly, four people lose their lives every day to asthma attacks.1
But the impact of uncontrolled asthma is not limited to these critical events and the strain on resources.1,2,5 Uncontrolled asthma also diminishes a patient’s health-related quality of life, negatively impacting many dimensions of a patient’s life, including mental function, depression, and distress.5
Despite these sobering statistics,1–3,5 many patients, and even healthcare professionals (HCPs), overestimate how well the condition is controlled, particularly in more severe asthma.6 In a cross-sectional UK study, ~84% of patients and ~74% of HCPs believed that asthma was controlled, however, asthma control test (ACT) results suggest actual control in only ~55% of patients (ACT score ≥20).6
Perceptions of asthma control6
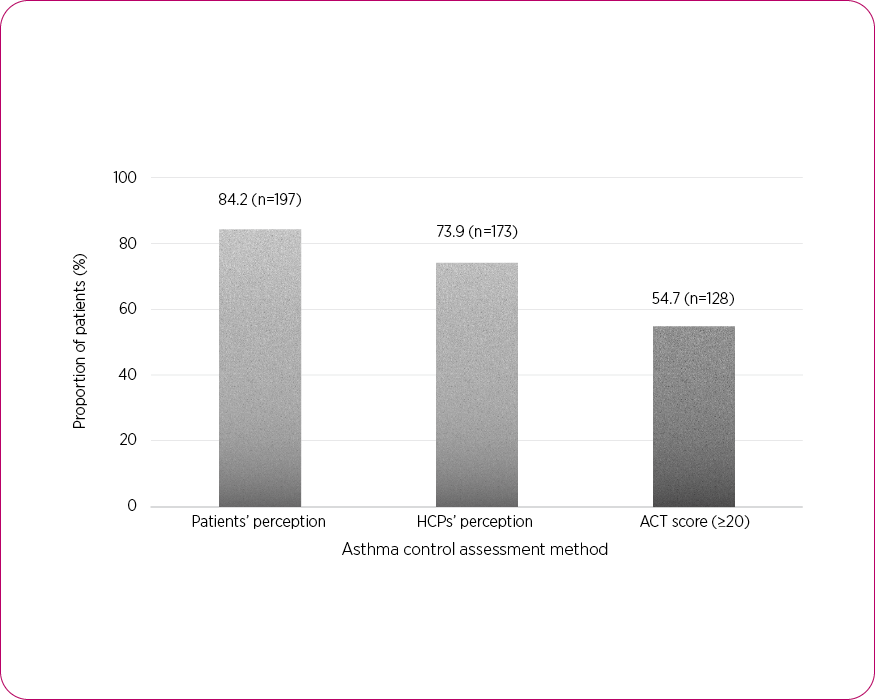
Adapted from Menzies-Gow A, et al. 2017.6
Achieving complete asthma control in patients’ everyday lives requires a critical re-evaluation of current treatment strategies – the most effective regimen requires thoughtful selection of both the medication and delivery device.7
The NEWTON study: real-world impact of Fostair NEXThaler 100/64
The NEWTON study was a multinational, observational, prospective cohort study evaluating Fostair NEXThaler 100/6 in adults with not well-controlled or poorly controlled asthma (n=423).4
The NEWTON study provides real-world evidence on this treatment, reporting improvements in asthma control (primary endpoint), moderate-severe exacerbation rate, lung function, quality of life (QoL) and treatment adherence vs. baseline. ~80% (n=307) of patients were generally satisfied with the NEXThaler device.4
Rapid and significant asthma control outcomes reported4,8
Two-thirds (66%; n=261/395; 95% CI: 61.2 to 70.7) of patients who completed the asthma control questionnaire (ACQ-5) reported achieving improved asthma control within six months of treatment with Fostair NEXThaler 100/6 compared to baseline, a key finding emphasising clinical effectiveness (primary endpoint).
Asthma control4
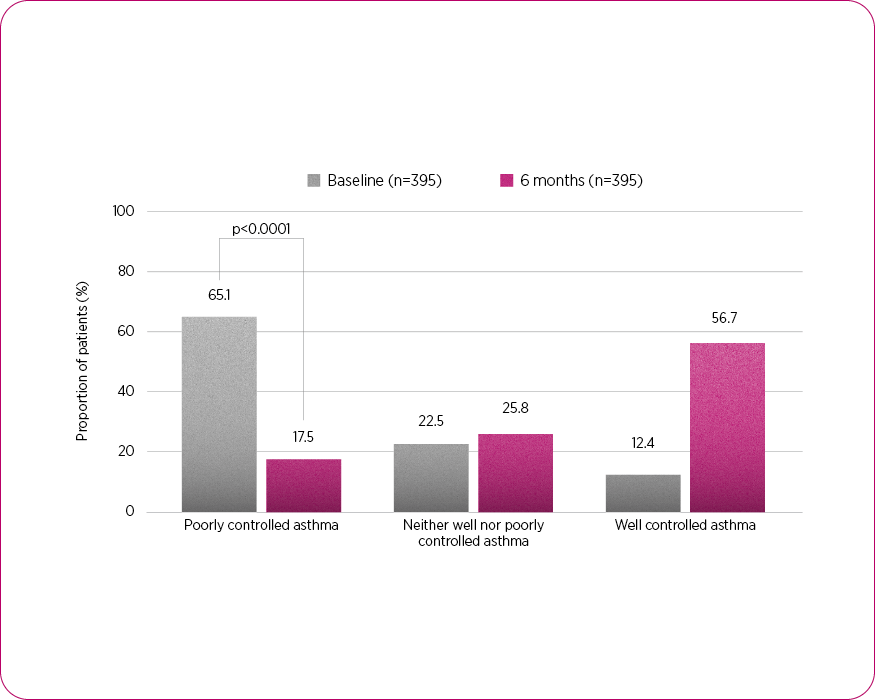
Adapted from Braido F, et al. 2025.4
The study also showed (secondary endpoint):4
- The proportion of patients with not-well-controlled or poorly controlled asthma was more than halved, dropping from 87.6% (n=346) to 43.3% (n=171) in six months
- Gains in asthma control were observed rapidly before stabilising, with similar positive results already evident at three months (median ACQ-5 decreased from 2.2 at baseline vs. 0.8 at 3 months; p<0.0001).
Explore the full study paper.
Further key findings from the NEWTON study
-
Significant improvements in lung function outcomes vs. baseline4
After six months, patients showed statistically significant improvements across key lung function parameters when taking Fostair NEXThaler 100/6. This included improvements in forced expiratory volume in one second (FEV1), forced vital capacity (FVC), mean forced expiratory flow between 25% and 75% of FVC (FEF25%-75%), and peak expiratory flow (PEF) at six months vs. baseline (p<0.0001 for all)* (secondary endpoint).
*Key lung function parameters included median FEV1 (% of predicted; n=150), FVC (% of predicted, n=151), FEF25%-75% (% of predicted, n=100), and PEF (% of predicted, n=125).4
-
Significant improvements in QoL vs. baseline4
NEWTON demonstrated a significant improvement in patients’ QoL with Fostair NEXThaler 100/6 vs. baseline, as assessed by the EuroQol 5-dimension 5-level (EQ-5D-5L) index. This improvement was evident within three months (n=354), with patients reaching a median interquartile range score of 1 (0.913-1.000) (indicating full health) by six months (n=391) (p<0.0001) (secondary endpoint).
-
In a sub-group analysis, more patients achieved ‘good adherence’ vs. baseline4
NEWTON reported notable improvements in treatment adherence with Fostair NEXThaler 100/6 vs. baseline. The proportion of patients demonstrating ‘good adherence’ increased significantly, rising from 31.7% (n=99) to 50% (n=156) at three months, and from 30.4% (n=105) to 49.9% (n=172) at six months from baseline with Fostair NEXThaler 100/6 (secondary endpoint).
Treatment adherence4
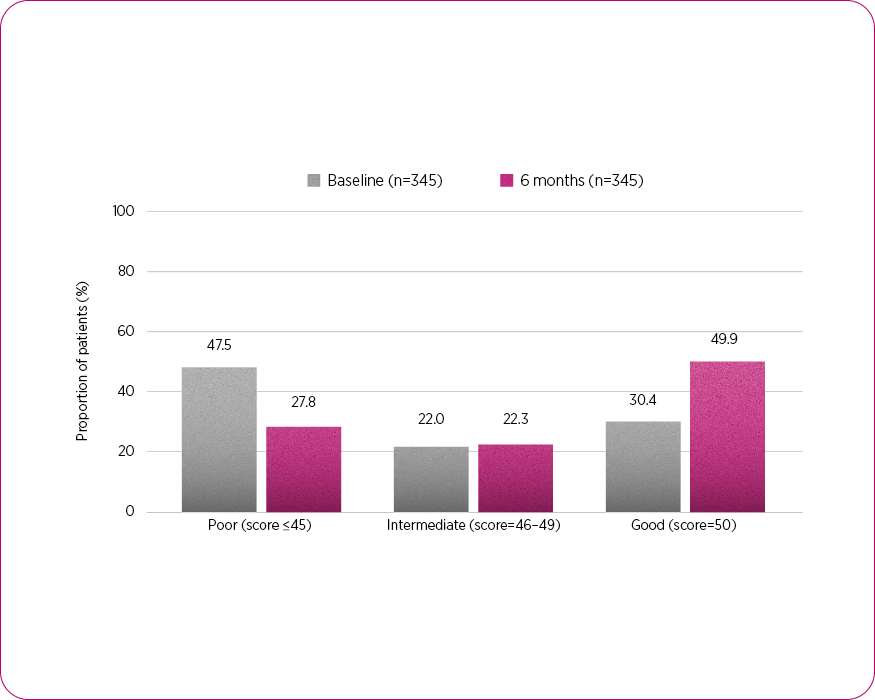
Adapted from Braido F, et al. 2025.4
-
Patients were satisfied with the Fostair NEXThaler 100/6 device4
Patient satisfaction with the NEXThaler device was high after six months of treatment (secondary endpoint). The majority of patients (n=387) reported being completely or very satisfied with:
● Its ease of use (81.1%) (n=314)
● The delivered dose check (83.7%) (n=324)
● And the device in general (79.3%) (n=307)These positive feelings were consistently observed even at three months, highlighting user acceptance and experience.
-
Safety profile and asthma exacerbation rate4
The study showed the tolerability of Fostair NEXThaler 100/6 was as expected, with no new safety concerns emerging. The most frequent treatment emergent adverse events (>2.0 %) were infections and infestations (3.3 %, n = 20), respiratory events (2.1 %, n = 13) and nervous system disorders (2.0 %, n = 12).
Importantly, in the 12 months before the enrollment 79 subjects (13.0 %) of the Safety Analysis Set population experienced moderate-to-severe exacerbations. At the end of observation, 21 subjects (3.4 %) experienced moderate-to-severe exacerbations, showing an improvement in disease stability.
Key takeaway
The persistent burden of uncontrolled asthma in the UK demands timely and effective intervention for these patients.1–3,5 Fostair NEXThaler 100/6 may provide significant benefits,8 now with demonstrated asthma control in the real world for the first time.4
To learn more about how Fostair NEXThaler 100/6 can help your patients with asthma achieve the control they need, speak to your Chiesi representative today.









